
OR
Nepal grants conditional emergency approval for COVID vaccine manufactured in India
Published On: January 15, 2021 05:24 PM NPT By: Republica | @RepublicaNepal
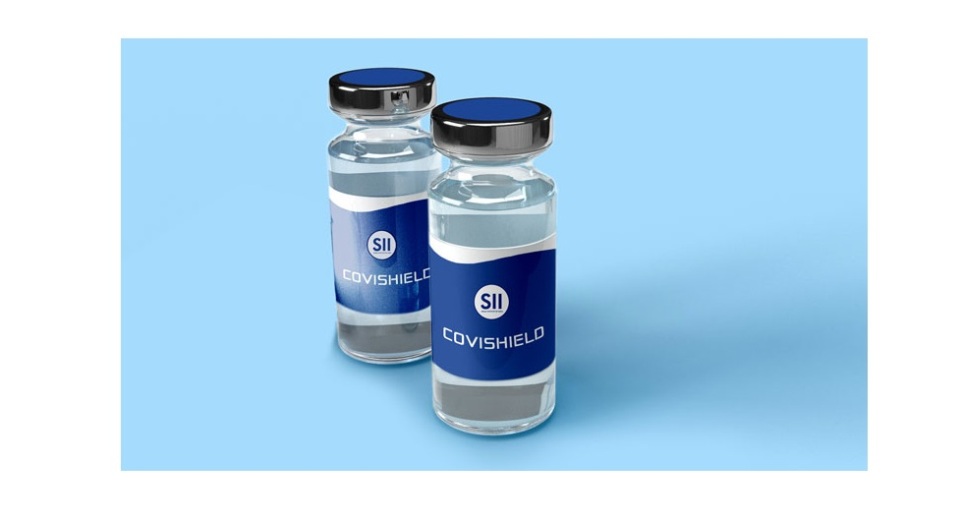
KATHMANDU, Jan 15: Nepal has granted a conditional emergency approval for Covishield, the coronavirus vaccine developed by AstraZeneca Plc and the University of Oxford, and manufactured by the Pune-based Serum Institute of India, the first step in its plan to inoculate citizens in the country.
A decision to grant the emergency approval for Covishield was taken on Friday itself, according to a press statement issued by the Department of Drug Administration this afternoon.
"Conditional permission has been granted for emergency use authorization of Covishield vaccine against COVID-19 in Nepal," reads the statement issued by the department.
In a notice on Wednesday, the department asked manufacturers or importers to get approval for registration in order to bring vaccines against COVID-19 in Nepal.
As of today, three importers have filed applications for the same, officials at the department stated.
Neighboring India has already made conditional emergency approval for Covishield manufactured by the Serum Institute.
Nepal has recorded 266,816 cases of coronavirus as of today.
You May Like This
Second drive of COVID-19 vaccination kicks off; 300,000 doses to be administered
KATHMANDU, Feb 8: The Ministry of Health and Population (MoHP) has rolled out the second phase of vaccination drive against... Read More...
India's Serum Institute seeks emergency use nod for AstraZeneca's COVID-19 vaccine - local media
INDIA, Dec 7: Serum Institute of India, the world’s largest vaccine producer by volume, has sought emergency use authorization in... Read More...
Vaccination drive against COVID-19 to start tomorrow from 62 hospitals and 120 vaccination centers
In the first phase, each of the around 450,000 frontline workers will get two doses of ‘Covishield’ vaccines ... Read More...






Just In
- Two climbers from Mongolia go missing during Everest expedition
- Police fires bullets to subdue drug dealer in Morang
- Govt forms Republic Day Main Celebration Committee
- EU delegation in Nepal to organize EU-Nepal Business Forum 2024
- Govt proposes to scrap or postpone ‘unnecessary’ infrastructure projects
- Koshi Province lawmaker Nirmala Limbu joins Rai-led JSP
- Director General of Department of Money Laundering Investigation fails to submit property details
- Historian Gyanmani Nepal passes away













Leave A Comment